- Background
Ocean acidification is acid formation when the ocean absorbs high levels of carbon dioxide (CO₂) gases from the atmosphere. The ocean helps balance Earth’s climate by absorbing CO₂ produced due to human activities, emitting sudden increases in CO₂ emissions from the burning of fossil fuel, among other sources. This balance tends to be broken (National Oceanic and Atmospheric Administration, 2020). Essentially, when CO₂ dissolves in seawater, it reacts with water to form carbonic acid, consequently lowering the ocean’s pH level. While the pH level for the ocean had always hovered around a slightly basic pH average of 8.2, it has now dropped to 8.1 since the Industrial Revolution, representing a 30% increase in acidity (European Environment Agency, 2023).
Acidification of the ocean is a major threat to marine ecosystems. The organisms that utilize calcium carbonate in forming their shells and skeletons, including corals, mollusks, and some species of plankton, are highly affected by the acidification of the ocean (National Oceanic and Atmospheric Administration, 2020). Increased acidity makes it difficult for these organisms to build up and maintain their structure, leading to weaker shells and coral reefs. Destroying coral reefs leads to completely eroding marine biodiversity (Allemand & Osborn, 2019).
b. Rain is Acidic lab experiment:
- You will need freshly collected rainwater, and a digitized pH meter. The rainwater should be collected in an open area away from the contaminations.
- Using the pH meter, measure the approximate pH level of the rainwater collected.
- With the aid of the same pH meter, measure the levels of pH of distilled water.
- Compare the pH values measured in steps 2, and 3 above.
c. What is changing that causes ocean acidification if rain is acidic itself
The major cause of ocean acidification is the absorption of atmospheric CO₂. Acid rain, generated by the pollution of sulfur and nitrogen oxides from industrial emissions, contributes to acidification of the ocean (National Oceanic and Atmospheric Administration, 2020). However, the key contributors to ocean acidification include the increased concentration of atmospheric CO₂, emanating from various human activities that involve burning fossil fuels, deforestation, and industrial processes. Dissolved atmospheric CO2 in seawater forms carbonic acid, which then dissociates to form hydrogen ions and bicarbonate (National Oceanic and Atmospheric Administration, 2020). This acid increases hydrogen ions’ concentration, decreasing pH and increasing the ocean’s acidity. Ocean acids are thus majorly due to carbon emissions, even though acidic rain has an impact.
d. The consequences of ocean acidification
i. Case Study: Coral Bleaching (Coral Reefs – Cebu, Philippines)
The case study presents Ebu, a coral triangle west of the Pacific Ocean. The region covers coral reefs across the Philippines, the Solomon Islands, Papua New Guinea, Timor-Leste, Malaysia, and Indonesia in the Pacific and Indian Oceans. It expounds on the economic benefits of coral reefs, including providing sand for beaches. These spectacular landscapes attract tourists who bring in much-needed income for local people and protect coastlines from strong currents, waves, and tropical storms. In addition, Reef fish feed the local populations with a valuable source of protein. However, the coral reefs have been threatened by many people visiting them for various leisure activities. The most direct threat to this coral colony has been ocean acidification from spills from powerboats and jet skis, affecting coral communities and the fish living among the reefs.
ii. Death of many marine specie
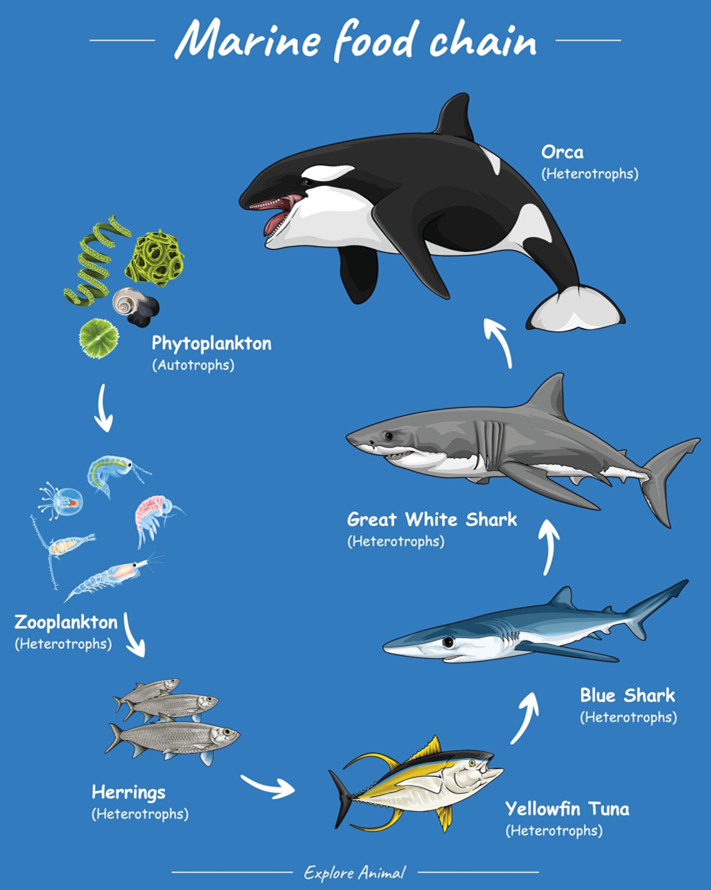
These deaths have been mostly associated with unprecedented changes in the pH of marine life due to oceanic acidification. These pH changes negatively affect phytoplankton and zooplankton, which are at the base of marine food chains. Many planktonic species build protective coverings composed of calcium carbonate, which is destroyed by increasing acidification. Furthermore, the marine food web relying on these plankton species as a primary source of food, from fish and birds to mammals, is destroyed. Species like oysters, clams, and scallops, which use calcium carbonate to make shells, are very sensitive to acidification, hence poor harvests among aquaculture industries. Mass death of marine species would disrupt these ecosystems and lead to a significant loss in biodiversity.
iii. Weakening of crustacean shells
Crustaceans, including crabs, lobsters, and shrimp, use calcium carbonate to form their exoskeleton. Ocean acidification, however, reduces the amount of carbonate ions, making it more difficult for these animals to develop strong shells. Weakened shells lead to the vulnerability of crustaceans to predation, disease, and environmental stress. Weakened shells contribute to lower survival rates, smaller populations, and fewer harvests.
e. How can we help?
1. Current efforts by organizations
Ocean acidification is an environmental issue that many organizations are engaged in the fight. The International Ocean Acidification Initiative at the Ocean Foundation leads in research, education, and policy development, while the Global Ocean Acidification Observing Network (GOAN) has been observing and instituting changes in oceanic chemistry worldwide.
2. Donating money
Financial aid Support Marine conservations, such as the Ocean Foundation’s Ocean Acidification Program, Marine Conservation Institute, and Ocean Conservancy have been providing finical aids on this venture. These funds can be used in the restoration projects in different ways and thereby protect the root causes of ocean acidification in marine ecosystems. Fund research initiatives can be channeled to research institutions studying ocean chemistry, carbon capture technologies, or alternative energy solutions to create a scientific understanding of how to mitigate acidification and develop ways to restore affected ecosystems.
3. Taking direct action
Individual action against ocean acidification is very critical. People living around the oceans need to reduce their carbon footprint by using renewable energies, among other initiatives. Furthermore, increased planting of trees helps release carbon dioxide from industries and other human-based activities. Other direct actions include beach cleanups, raising awareness about the growing acidity in our ocean, supporting policies that move us toward reducing emissions, and even eco-friendly gardening to prevent pollutants from pouring into our storm drains and out into our oceans. With these improvements to mitigate acidification and develop ways to restore affected ecosystems, the marine environments will be significantly improved and conserved.
References
Allemand, D., & Osborn, D. (2019). Ocean acidification impacts on coral reefs: From sciences to solutions. Regional Studies in Marine Science, 28(28), 100558. https://doi.org/10.1016/j.rsma.2019.100558
European Environment Agency. (2023, April 28). Ocean acidification. Www.eea.europa.eu. https://www.eea.europa.eu/en/analysis/indicators/ocean-acidification
National Oceanic and Atmospheric Administration. (2020, April 1). Ocean acidification | National Oceanic and Atmospheric Administration. Www.noaa.gov. https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification#:~:text=Carbon%20dioxide%20and%20seawater&text=Water%20and%20carbon%20dioxide%20combine